The medical community’s understanding and approach to essential tremor (ET) have evolved significantly over the years. Central to this evolution are clinical trials, which serve as the backbone for validating new treatments and interventions. This article delves into the importance of clinical trials in the realm of essential tremor management and how they pave the way for innovations like the Steadi-Two glove.
Table of Contents
The Essence of Clinical Trials
Clinical trials are structured research studies that test the safety and effectiveness of new medical interventions, be it drugs, devices, or treatment protocols. These trials are meticulously designed, ensuring that the results obtained are reliable and can be generalized to a broader population.
For conditions like essential tremor, clinical trials provide invaluable insights into the potential benefits and limitations of new treatments. They offer a structured framework to test hypotheses, validate results, and ensure that any new intervention meets the rigorous standards set by regulatory bodies.
Recent Breakthroughs in Essential Tremor Trials
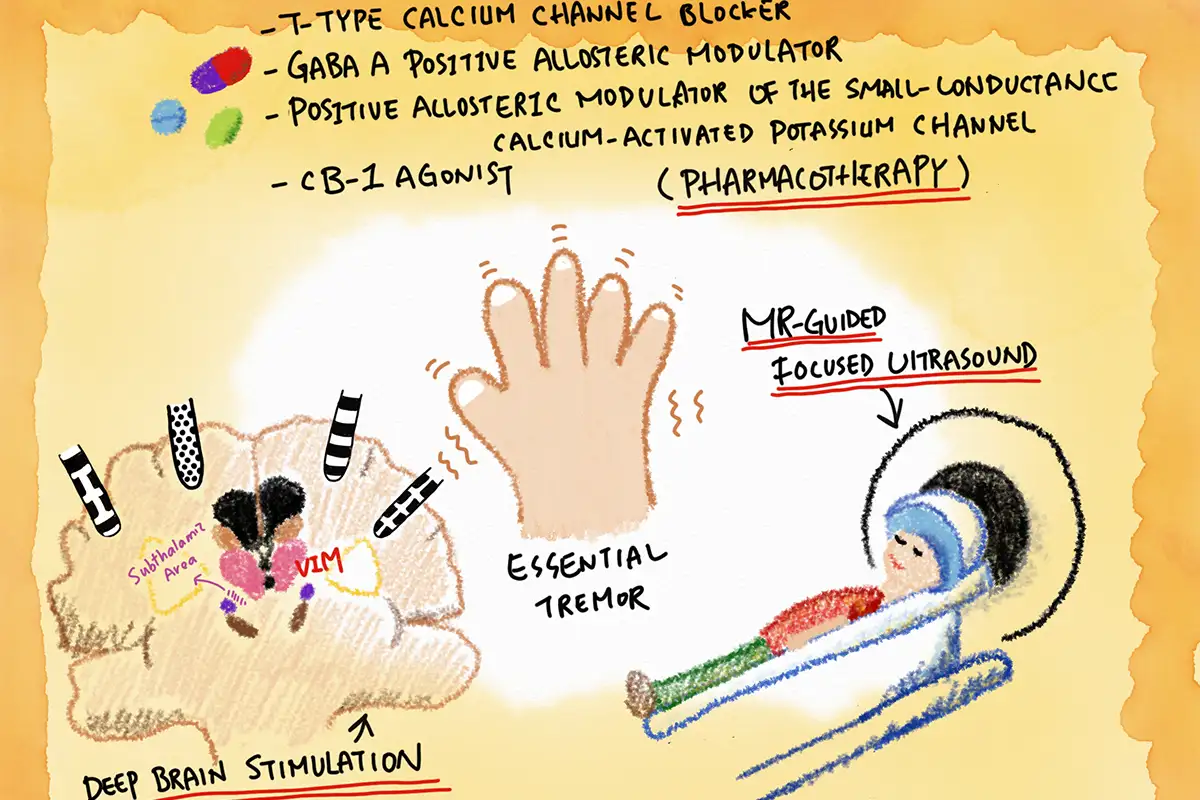
One of the notable clinical trials in recent times is the Phase 2 Essential1 clinical study of ulixacaltamide by Praxis Precision Medicines. The trial aimed to evaluate the drug’s efficacy in treating essential tremor. The positive results from this trial not only highlighted the potential of ulixacaltamide but also underscored the importance of clinical trials in bringing such innovations to light.
Furthermore, several other trials are ongoing, exploring various facets of essential tremor management. From understanding voice control in ET patients to evaluating the safety and efficacy of new drugs, these trials are at the forefront of medical advancements in the field.
Steadi-Two: Backed by Research and Feedback
While the Steadi-Two glove by Steadiwear is a non-pharmacological solution, its design and efficacy are rooted in extensive research and user feedback. Before its launch, the glove underwent multiple iterations, with each version refined based on user experiences and feedback.
Clinical studies and user trials helped Steadiwear understand the glove’s impact on daily activities, its comfort level, and its overall effectiveness in reducing tremor amplitude. Such a structured approach ensured that the final product was not only technically sound but also catered to the real-world needs of ET patients.
The Community’s Role in Clinical Trials
The success of clinical trials is not solely dependent on researchers and medical professionals. The community plays a pivotal role. Participation from individuals with essential tremor is crucial to gather diverse data, understand the varied impacts of interventions, and refine treatments based on real-world experiences.
Organizations and companies often encourage ET patients to participate in trials, ensuring that the results are representative and comprehensive. Such collaborative efforts between the medical community and patients are instrumental in driving advancements in ET management.
Ethical Considerations in Clinical Trials
While the primary objective of clinical trials is to advance medical knowledge and improve patient care, they must be conducted ethically. Participants’ rights, safety, and well-being are paramount. Informed consent, where participants are made fully aware of the trial’s purpose, procedures, potential risks, and benefits, is a fundamental requirement.
For conditions like essential tremor, where the impact on daily life can be profound, it’s crucial that participants are not given false hope or subjected to unnecessary risks. Ethical oversight, often provided by Institutional Review Boards (IRBs), ensures that trials are conducted with integrity, respect, and fairness. This ethical foundation not only protects participants but also lends credibility to the trial’s findings.
The Evolution of Non-Invasive Treatments in Clinical Trials
Traditionally, clinical trials have heavily focused on pharmacological solutions. However, with the rise of technology and a better understanding of conditions like essential tremor, there’s a growing interest in non-invasive treatments. Devices like the Steadi-Two glove represent this shift in focus.
Trials evaluating non-invasive solutions offer a different set of challenges and opportunities. For instance, the parameters for measuring efficacy might differ from drug trials. Instead of biochemical markers, researchers might focus on functional improvements or quality of life metrics. The rise of wearable technology, AI-driven analysis, and real-time feedback mechanisms are also shaping how non-invasive treatments are evaluated in clinical settings.
The Global Landscape of Essential Tremor Research
Essential tremor is not just a localized concern; it’s a global challenge that affects millions worldwide. Research and clinical trials are being conducted across continents, with each region bringing its unique perspective and approach to the table. For instance, while North America might be leading in technological interventions like the Steadi-Two glove, Europe might be focusing on genetic studies related to ET. Asia, with its vast population, offers a diverse patient pool that can provide invaluable insights into the condition’s varied manifestations. Collaborative international studies can harness these regional strengths, leading to more holistic and effective solutions for ET.
The Role of Technology in Enhancing Clinical Trials
With the advent of digital health platforms, telemedicine, and wearable sensors, the way clinical trials are conducted is undergoing a transformation. Remote monitoring allows for real-time data collection, ensuring that any adverse reactions are promptly addressed. Moreover, digital platforms enable wider participation, as patients from remote areas can now be part of trials without the need for frequent hospital visits. This digital shift not only makes trials more efficient but also more inclusive, capturing a broader spectrum of patient experiences.
Patient Advocacy and Awareness Initiatives
While clinical trials are crucial for medical advancements, patient advocacy and awareness play an equally vital role in shaping the future of ET management. By raising awareness about the condition, more individuals can be diagnosed early, leading to better management and improved quality of life. Patient advocacy groups also play a pivotal role in pushing for more research funding, ensuring that ET remains a priority in the medical community. These groups serve as a bridge between patients and researchers, ensuring that the latter’s work remains aligned with the real-world needs of ET patients.
In conclusion, clinical trials are the cornerstone of medical advancements in essential tremor management. They provide the necessary validation for new treatments, ensuring they are safe and effective. With continued research, collaboration, and community participation, the future of essential tremor management looks promising, offering hope and improved quality of life to those affected by this condition.